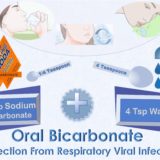
Oral Bicarbonate For Protection From Respiratory Viral Infection
Thousands Of Respiratory Virus Particles Are Inhaled In Every Breath 1
Micro-organisms are everywhere around us and within us. Small in size, located both inside and outside natural human cells. Acquired shortly after birth, they aid digestion and metabolism, provide protection from pathogenic organisms and inhibit viral infection.
Fortunately, fewer than 1% of all the microbes people contact are potentially dangerous. Most healthy individuals with normal immunity escape dangerous microbial infection.
Most particles that are inhaled are exhaled in the same breath. But a few are trapped in the mucosal epithelial lining of mouth, nose and throat. Most that are trapped and recognized as pathogens are destroyed by protective blood and tissue cells.
Respiratory Viral Infection Requires Acid Oral Saliva 2
 Salivary glands are very important. The fluid they secrete protects teeth, starts digestion and helps swallowing.
Salivary glands are very important. The fluid they secrete protects teeth, starts digestion and helps swallowing.
Enamel covering and protecting teeth is the hardest structure in the body. More dense even than bone. But acid softens it and lets calcium escape from the surface of teeth.
Acid damages enamel, alkali repairs it. Ideally, the more alkaline, the better. Alkaline levels in the mouth help to remineralize enamel and fight against cavity-causing bacteria.
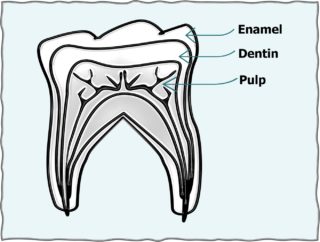
Almost all eating and drinking brings acid into the mouth. The acid-base balance of oral fluid is measured in 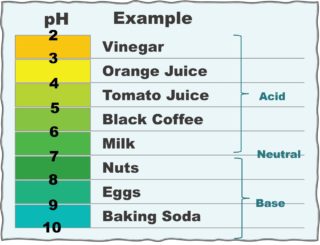 pH units. Increase and decrease of pH is opposite (inverse) to the power (concentration) of Hydrogen (H+) ions relative to Hydroxyl (OH-) ions in water (HOH or H2O).
pH units. Increase and decrease of pH is opposite (inverse) to the power (concentration) of Hydrogen (H+) ions relative to Hydroxyl (OH-) ions in water (HOH or H2O).
Perfect balance between H+ and OH- is assigned a pH of 7.0. Thus, 6.9 is acidic and 7.1 is alkaline. Ideal blood pH is 7.4 with a normal range of 7.35 to 7.45. In general, abnormal metabolism in human cells tends to release H+ ions and lower the pH.
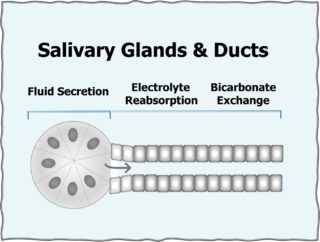 Most of saliva is secreted by acinar cells and then modified in the ducts. Proteins are added to fluid from serum of blood flowing through the glands. Enzymes act to produce bicarbonate in the ducts and start digestion in the oral saliva. The pH of ductal saliva is low when flow is low and increases as ductal flow of saliva increases.
Most of saliva is secreted by acinar cells and then modified in the ducts. Proteins are added to fluid from serum of blood flowing through the glands. Enzymes act to produce bicarbonate in the ducts and start digestion in the oral saliva. The pH of ductal saliva is low when flow is low and increases as ductal flow of saliva increases.
Oral Microbiome 3
There are millions of bacteria on tongue and teeth and in oral mucosa and saliva. Hundreds of different kinds but with a similar core of functional types in all individuals. Differences in each individual depend on a combination of diet, lifestyle and genetic determinants. All representing a balance between competing processes for growth and function.
 The microbiome provides functions that human cells have never developed. Functions related to digestion, metabolism, immunity and tissue cell maintenance. Good health ultimately depends on communication and harmony between bacteria and natural human cells. The best possible coordination results in mildly alkaline oral saliva.
The microbiome provides functions that human cells have never developed. Functions related to digestion, metabolism, immunity and tissue cell maintenance. Good health ultimately depends on communication and harmony between bacteria and natural human cells. The best possible coordination results in mildly alkaline oral saliva.
The oral microbiomes of individuals in good health are much alike according to age, sex, diet and body composition. Good health has balanced actions for normal function. Secreting water, proteins, electrolytes and bicarbonate that keep the range of pH between 6.7 and 7.4, making oral saliva relatively neutral.
However, acid saliva is dangerous for risk of respiratory viral infection even when health of mouth and body is good. The task for safe saliva is to keep pH of oral fluid 7.0 or higher.
Saliva from acinar cells is very dilute and mildly acidic. Those cells secrete enzymes that transfer chloride for bicarbonate in cells along the ducts to the mouth. Bicarbonate ions increase pH of fluid reaching the mouth.
Increasing alkalinity of oral fluids favors growth of cells that produce bicarbonate and ammonium ions. Those bacteria enormously enhance alkalinity of oral saliva. The result is suppression of acid-producing bacteria functioning in oral saliva.
Nasal Irrigation and Oral Rinse 4
Oral and nasal saline irrigation has long been used for the treatment of upper respiratory viral infections. The procedure involves flushing the nasal cavity and rinsing the oropharyngeal region with saline solution. The treatment relieves symptoms and reduces viral load.
The combination of bicarbonate and saline solutions is more effective than sodium chloride alone.
Rapid Initiation Of Nasal Saline Irrigation 5
The effects of nasal irrigation have been tested treating outpatients with COVID-19. A clinical trial of high-risk patients was conducted in Georgia, USA. Patients recruited were recently positive for SARS-CoV-2 virus in nasal swabs or saliva. 79 participants were randomly assigned to twice-daily nasal irrigation.
The patients reporting twice daily irrigations with sodium bicarbonate solution were more than 8 times less likely to be hospitalized than patients not receiving nasal irrigation. 6
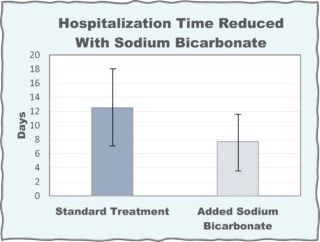 Treatment of hospitalized patients with mild or moderate COVID-19 has also been tested. In a Chinese hospital, 55 patients were divided into two groups. Both groups received regular care with the experimental group also receiving nasal irrigation and oral rinse with 5% sodium bicarbonate solution. Oral and nasopharyngeal swab samples were collected daily for assays of viral content. The negative conversion time and hospitalization time of the patients were recorded. As shown in the Figure, the average hospitalization time was 12.5 days for the control group and 7.7 days for the experimental group.
Treatment of hospitalized patients with mild or moderate COVID-19 has also been tested. In a Chinese hospital, 55 patients were divided into two groups. Both groups received regular care with the experimental group also receiving nasal irrigation and oral rinse with 5% sodium bicarbonate solution. Oral and nasopharyngeal swab samples were collected daily for assays of viral content. The negative conversion time and hospitalization time of the patients were recorded. As shown in the Figure, the average hospitalization time was 12.5 days for the control group and 7.7 days for the experimental group.
Oral Bicarbonate 7
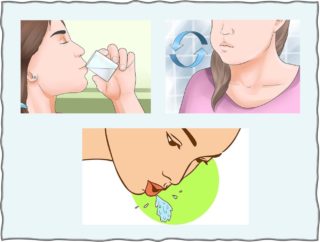 Oral bicarbonate in a mouthwash greatly increases pH of oral saliva. Rinsing with 6% solution of baking soda without swallowing enhances growth and metabolism of alkali-producing bacteria. Increase in function of those bacteria favors continued production of bicarbonate even after ridding the mouthwash.
Oral bicarbonate in a mouthwash greatly increases pH of oral saliva. Rinsing with 6% solution of baking soda without swallowing enhances growth and metabolism of alkali-producing bacteria. Increase in function of those bacteria favors continued production of bicarbonate even after ridding the mouthwash.
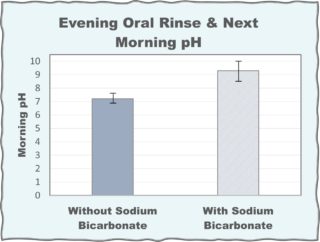 Effect of treatment with oral bicarbonate rinse at night on pH of resting saliva in the next morning has been tested. A group of 25 healthy young adult subjects from Era Medical College in India were tested before and after alkalizing oral rinse at night. The average morning resting saliva pH was 7.2 without alkalizing oral rinse the previous night. After alkalizing treatment the night before, the morning resting saliva pH was 9.4.
Effect of treatment with oral bicarbonate rinse at night on pH of resting saliva in the next morning has been tested. A group of 25 healthy young adult subjects from Era Medical College in India were tested before and after alkalizing oral rinse at night. The average morning resting saliva pH was 7.2 without alkalizing oral rinse the previous night. After alkalizing treatment the night before, the morning resting saliva pH was 9.4.
 Oral bicarbonate that is swallowed, increases levels of bicarbonate in serum. That reduces the effect of organic acids circulating in blood. As a result, level of organic acids in fluid secreted by acinar cells is reduced and pH of oral saliva is increased.
Oral bicarbonate that is swallowed, increases levels of bicarbonate in serum. That reduces the effect of organic acids circulating in blood. As a result, level of organic acids in fluid secreted by acinar cells is reduced and pH of oral saliva is increased.
Treatment With Sodium Bicarbonate
The standard dose of sodium bicarbonate for Oral Rinse and Oral Ingestion is 1 to 3 grams/day taken at night, several hours after meals and snacks.
The Oral Rinse with 1.5 grams in a 5% solution is prepared by dissolving 1/4 teaspoon of powdered sodium bicarbonate (Baking Soda) in 4 teaspoons of water. The rinse with about 1 ounce of solution is swished around in the mouth for 30 to 60 seconds and then expectorated.
The Oral Ingestion is prepared by dissolving 1/4 teaspoon of powdered sodium bicarbonate (Baking Soda) in 2 ounces of water. The solution is swallowed completely.
Tablets of sodium bicarbonate contain 325 or 650 mg. Capsules contain 500 or 1600 mg.
Summary
Sodium bicarbonate is a naturally formed substance in tissue and blood that enables acid-base balance. The bicarbonate combines with H+ and then dissociates to CO2 and water. Release and retention of CO2 in the lungs and retention and excretion of bicarbonate ion in kidneys maintains bodily functions in a tight range of pH.
The evolution of living cells during billions of years involves close association and interactive function between animal cells and microbes. As a result, normal function of human cells depends on interaction with bacteria.
 Millions of bacteria live all around us and inside us. Only a few of them are dangerous and natural functions protect us from most harmful infections.
Millions of bacteria live all around us and inside us. Only a few of them are dangerous and natural functions protect us from most harmful infections.
Respiratory viral infections cause the most trouble. Our best protection from respiratory infection depends on maintaining alkaline secretions by salivary, oropharyngeal and nasal cells. Some of the protection arises from normal, healthy function. But even normal health is not completely protective. Acid secretions are particularly harmful.
Sodium bicarbonate in an oral rinse at night increases oral pH and enhances continuing bicarbonate production by oral bacteria after salivary secretion of bicarbonate by salivary cells decreases during sleep. Alkaline oral fluid protects cells of mouth, nose and throat from becoming infected by respiratory viruses.
Sodium bicarbonate swallowed at night increases the level of bicarbonate in serum circulating through cells of mouth, nose and throat all night. Maintaining alkaline pH in cells and mucosal fluid around them protects cells from infection by respiratory viruses.
Alkaline fluid protecting tissue cells from infection also enables recognition and destruction of pathogens in blood and extracellular fluid.
Let’s See If We Can Help You
We’ll send supplies for testing to the first 50 people who request our help.
When you send us results of your testing for 5-7 days, we’ll analyze risks those data indicate. We’ll call and help you maintain health and reduce risks from infection.
[Disclaimer: It’s important to consult with your Primary Care Physician to ensure accuracy and alignment with your individual medical condition and treatment.]
______________________________________________________________________________________________________
REFERENCES
1. Wang CC, Prather KA, et al. Airborne transmission of respiratory viruses. Science, 2021;373(6558). https://doi.org/10.1042/BJ20051920
2. Kreutzberger AJ, Sanyal A, et al. SARS-CoV-2 requires acidic pH to infect cells. Proceedings of the National Academy of Sciences, 2022;119(38). https://doi.org/10.1371/journal.ppat.1004294
3. Pedersen AML, Belstrøm D. The role of natural salivary defences in maintaining a healthy oral microbiota. Journal of dentistry, 2019;80, S3-S12. https://doi.org/10.1152/physrev.00030.2019
4. Principi N, Esposito S. Nasal irrigation: an imprecisely defined medical procedure. International journal of environmental research and public health, 2017;14(5),516. https://doi.org/10.1073/pnas.2209514119
5. Baxter AL, Schwartz KR, et al. Rapid initiation of nasal saline irrigation to reduce severity in high-risk COVID+ outpatients. Ear, Nose & Throat Journal, 2022;01455613221123737. https://doi.org/10.1073/pnas.2209514119
6. Wang T, Zhang Y, et al. Efficacy of nasal irrigation and oral rinse with sodium bicarbonate solution on virus clearance for COVID-19 patients. Frontiers in Public Health, 2023;11,1145669. https://doi.org/10.1073/pnas.2209514119
7. Chandel S, Khan MA, et al. The effect of sodium bicarbonate oral rinse on salivary pH and oral microflora: A prospective cohort study. National journal of maxillofacial surgery, 2017;8(2),106. https://doi.org/10.1073/pnas.2209514119